Buy high quality and low price Losartan 114798-26-4 now
- Molecular Formula:C22H23ClN6O
- Molecular Weight:422.917
- Appearance/Colour:pale yellow solid
- Vapor Pressure:8.63E-20mmHg at 25°C
- Melting Point:183-184 °C
- Refractive Index:1.68
- Boiling Point:682 °C at 760mmHg
- PKA:5-6(at 25℃)
- Flash Point:366.3 °C
- PSA:92.51000
- Density:1.35 g/cm3
- LogP:4.26680
Losartan(Cas 114798-26-4) Usage
|
Manufacturing Process
|
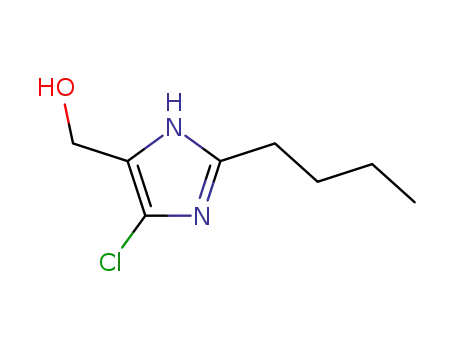
2-Butyl-4-chloro-1-(2'-(tetrazol-5-yl)biphenyl-4-ylmethyl)-1H-imidazole-5- methanolpotassium was synthesized in 5 stages. 1. Methyl 4'-methylbiphenyl-2-carboxylate (44.2 mmol), 0.5 N KOH in methanol (133 mmol), and water (50 mL) were mixed and refluxed under nitrogen. After 5 hours, the solvent was removed in vacuo and water (200 mL) and ethyl acetate (200 mL) added. The aqueous layer was acidified with concentrated hydrochloric acid to a pH of 3 and the layers were separated. The aqueous phase was extracted with ethyl acetate, the organic layers collected, dried (MgSO4) and the solvent removed in vacuo to yield 8.71 g of a 4'-methylbiphenyl-2-carboxylic acid, melting point 140.0-145.0°C. 2. 4'-Methylbiphenyl-2-carboxylic acid (41 mmol) and thionyl chloride (411 mmol) were mixed and refluxed for 2 hours. The excess thionyl chloride was removed in vacuo and the residue was taken up in toluene. The toluene was removed by rotary evaporation. The crude acid chloride was then added slowly to cold (0°C) concentrated NH4OH (50 mL) so that the temperature was kept below 15°C. After 15 minutes of stirring, water (100 mL) was added and solids precipitated. These were collected, washed with water and dried under high vacuum over P2O5 to yield 7.45 g of a white solid, melting point 126.0-128.5°C. The above product amide (35 mmol) and thionyl chloride (353 mmol) were mixed and refluxed for 3 hours. The thionyl chloride was removed using the same procedure as described above. The residue was washed with a little hexane to yield 6.64 g of 4'-methyl-2-cyanobiphenyl, melting point 44.0- 47.0°C. 3. 4'-Methyl-2-cyanobiphenyl (5.59 g) was brominated using benzoyl peroxide as an initiator. The product was recrystallized from ether to yield 4.7 g of 4'- bromomethyl-2-cyanobiphenyl, melting point 114.5-120.0°C.4. 4'-Bromomethyl-2-cyanobiphenyl (4.6 g) was alkylated onto 2-n-butyl-4-
chloro-5-(hydroxymethyl)-imidazole. For separation of the product was used a
flash chromatography in 1:1 hexane/ethyl acetate over silica gel. The
regioisomeric products yielded 2.53 g of the faster eluting isomer.
Recrystallization from acetonitrile yielded 1.57 g of analytically pure 2-n-butyl4-chloro-1-[2'-cyanobiphenyl-4-yl)methyl]-5-(hydroxymethyl)-imidazole,
melting point 153.5 -155.5°C.
5. 2-n-Butyl-4-chloro-1-[(2'-cyanobiphenyl-4-yl)-methyl]-5-(hydroxymethyl)-
imidazole (10 mmole), sodium azide (10 mmol), and ammonium chloride (30
mmol) were mixed in DMF (150 mL) under N2 at 100°C for 2 days, after
which the temperature was raised to 120°C for 6 days. The reaction was
cooled and 3 more equivalents each of ammonium chloride and sodium azide
were added. The reaction was again heated for 5 days at 120°C. The reaction
was cooled, the inorganic salts filtered, and the filtrate solvent removed in
vacuo. Water (200 mL) and ethyl acetate (200 mL) were added to the residue
and the layers were separated. The aqueous layer was extracted with ethyl
acetate, the organic layers were collected, dried (MgSO4) and the solvent
removed in vacuo, to yield a dark yellow oil. The product was purified by flash
chromatography in 100% ethyl acetate to 100% ethanol over silica gel to
yield 5.60 g of a light yellow 2-n-butyl-4-chloro-5-hydroxymethyl-1-[(2'-(1Htetrazol-5-yl)biphenyl-4-yl)methyl]imidazole. Recrystallization from acetonitrile
yielded 4.36 g of light yellow crystals which still melted broadly. The crystals
were taken up in 100 mL of hot acetonitrile. The solid that did not dissolve
was filtered off to yield 1.04 g of product as a light yellow solid, melting point
of 2-n-butyl-4-chloro-5-hydroxymethyl-1-[(2'-(1H-tetrazol-5-yl)biphenyl-4-
yl)methyl]imidazole 183.5-184.5°C.
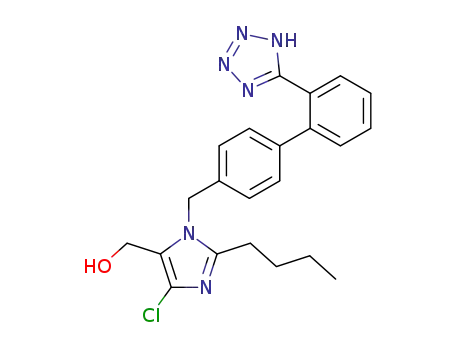
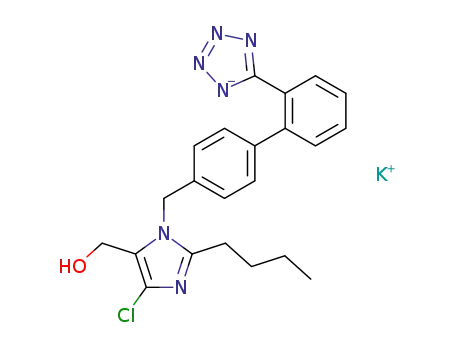
2-n-Butyl-4-chloro-5-hydroxymethyl-1-[(2'-(1H-tetrazol-5-yl)biphenyl-4-
yl)methyl]imidazole may be converted to potassium salt. |
|
Therapeutic Function
|
Antihypertensive |
|
Flammability and Explosibility
|
Nonflammable |
|
General Description
|
Losartan is an angiotensin II receptor antagonist, originally developed as DuP 753, that serves as a therapeutic agent for conditions such as hypertension by blocking the effects of angiotensin II. The study explored novel derivatives, including triazole, sulfur-containing diazole, and N-phenylthiatriazole biphenyltetrazoles, to identify compounds with comparable or improved efficacy, with one derivative showing promising in vitro activity. However, losartan remains a well-established reference in this class of medications.
**Final paragraph (conclusion):**
Losartan is a clinically effective angiotensin II receptor antagonist used primarily for hypertension management, with its structure serving as a basis for developing newer derivatives aimed at enhancing therapeutic potential. |
|
Definition
|
ChEBI: A biphenylyltetrazole where a 1,1'-biphenyl group is attached at the 5-position and has an additional trisubstituted imidazol-1-ylmethyl group at the 4'-position |
|
Brand name
|
Cozaar (Merck). |
InChI:InChI=1/C22H23ClN6O/c1-2-3-9-20-24-21(23)19(14-30)29(20)13-15-10-11-17(16-7-5-4-6-8-16)18(12-15)22-25-27-28-26-22/h4-8,10-12,30H,2-3,9,13-14H2,1H3,(H,25,26,27,28)
114798-26-4 Relevant articles
Novel and efficient debenzylation of N-benzyltetrazole derivatives with the rosenmund catalyst
Seki, Masahiko
, p. 3249 - 3255 (2014)
The Rosenmund catalyst (Pd/BaSO4) was fo...
Efficient synthesis of losartan, a nonpeptide angiotensin II receptor antagonist
Larsen,King,Chen,Corley,Foster,Roberts,Yang,Lieberman,Reamer,Tschaen,Verhoeven,Reider,Lo,Rossano,Brookes,Meloni,Moore,Arnett
, p. 6391 - 6394 (1994)
A highly efficient, convergent approach ...
Unusual detritylation of tritylated tetrazole in Sartan molecules
Srimurugan, Sankareswaran,Suresh, Paulsamy,Babu, Balaji,Hiriyanna, Salmara Ganeshbhat,Pati, Hari Narayan
, p. 383 - 384 (2008)
Tritylated tetrazole of 2a underwent unu...
Preparation method of losartan
-
Paragraph 0033-0042, (2021/04/21)
The invention provides a preparation met...
Method for preparing high-purity losartan
-
Paragraph 0020-0034, (2020/10/19)
The invention relates to a method for pr...
Preparation method of losartan
-
, (2019/11/29)
The invention provides a preparation met...
A trityl protecting group by removing method of preparing losartan medicine
-
Paragraph 0032-0039; 0047-0051, (2018/07/30)
The invention discloses a method for pre...
114798-26-4 Process route
-
-
133909-99-6
2-n-butyl-4-chloro-1-[(2'-(2-triphenylmethyl-2H-tetrazol-5-yl)-1,1'-biphenyl-4-yl)methyl]-1H-imidazole-5-methanol
-
-
76-84-6
triphenylmethyl alcohol
-
-
114798-26-4
2-n-butyl-4-chloro-5-hydroxymethyl-1-[(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]imidazole
Conditions
| Conditions |
Yield |
|
2-n-butyl-4-chloro-1-[(2'-(2-triphenylmethyl-2H-tetrazol-5-yl)-1,1'-biphenyl-4-yl)methyl]-1H-imidazole-5-methanol;
With
hydrogenchloride;
In
water; acetone;
at 20 ℃;
for 5h;
With
potassium hydroxide;
In
water; acetone;
Product distribution / selectivity;
|
|
|
2-n-butyl-4-chloro-1-[(2'-(2-triphenylmethyl-2H-tetrazol-5-yl)-1,1'-biphenyl-4-yl)methyl]-1H-imidazole-5-methanol;
With
sulfuric acid;
In
water; acetone;
at 25 ℃;
for 4.33333h;
Industry scale;
With
potassium hydroxide;
In
water; acetone;
at 18 - 25 ℃;
for 16.5h;
pH=13.3;
Product distribution / selectivity;
Industry scale;
|
|
-
-
124751-00-4
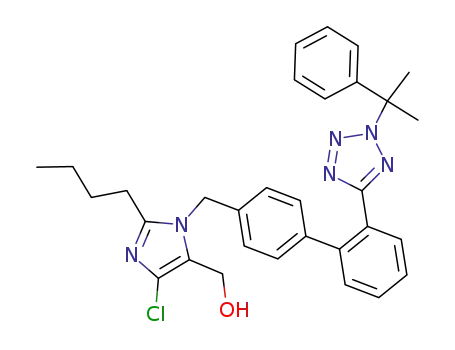
2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol
Conditions
| Conditions |
Yield |
|
2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol;
hydroxylamine hydrochloride;
In
methanol;
at 60 ℃;
for 2.5h;
pH=2.95;
With
triethylamine;
In
methanol;
at 0 - 40 ℃;
for 1h;
pH=3.6;
Product distribution / selectivity;
|
94.7%
|
|
2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol;
hydroxyammonium sulfate;
In
isopropyl alcohol;
at 60 - 65 ℃;
for 4h;
pH=2.4;
With
triethylamine;
In
isopropyl alcohol;
at 0 - 5 ℃;
for 2h;
pH=3.5;
Product distribution / selectivity;
|
93.8%
|
|
With
hydrogenchloride; water;
In
tetrahydrofuran;
at 20 ℃;
for 4h;
|
90%
|
|
With
TRILITE CMP-12 porous type;
In
methanol;
for 5h;
Product distribution / selectivity;
Reflux;
|
89%
|
|
With
sodium hydroxide;
In
methanol;
at 20 ℃;
|
87%
|
|
With
hydrogenchloride;
In
1,4-dioxane; water;
at 20 ℃;
|
67%
|
|
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 20 - 23 ℃;
|
|
|
2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol;
With
methanol; water;
toluene-4-sulfonic acid;
In
tetrahydrofuran;
at 20 ℃;
for 144h;
With
sodium hydroxide;
In
tetrahydrofuran; methanol; dichloromethane; water;
pH=12;
|
|
|
With
hydrogenchloride; sodium hydroxide; acetic acid;
In
methanol; water; ethyl acetate; toluene;
|
|
|
With
hydrogenchloride; sodium hydroxide; acetic acid;
In
methanol; water; ethyl acetate; toluene;
|
|
114798-26-4 Upstream products
-
133909-99-6
2-n-butyl-4-chloro-1-[(2'-(2-triphenylmethyl-2H-tetrazol-5-yl)-1,1'-biphenyl-4-yl)methyl]-1H-imidazole-5-methanol
-
852357-69-8
(2-butyl-5-chloro-3-{2'-[2-(1-methyl-1-phenyl-ethyl)-2H-tetrazol-5-yl]-biphenyl-4-ylmethyl}-3H-imidazol-4-yl)-methanol
-
79047-41-9
2-n-butyl-4-chloro-5-hydroxymethylimidazole
-
83857-96-9
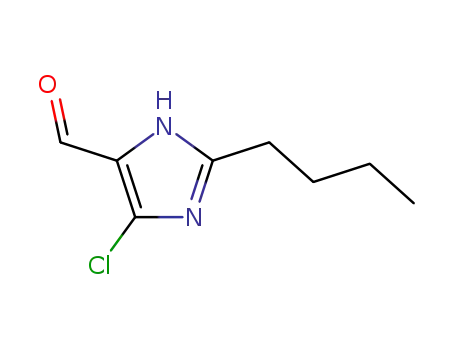
2-n-butyl-4-chloro-1H-imidazol-5-carboxaldehyde
114798-26-4 Downstream products
-
791122-48-0
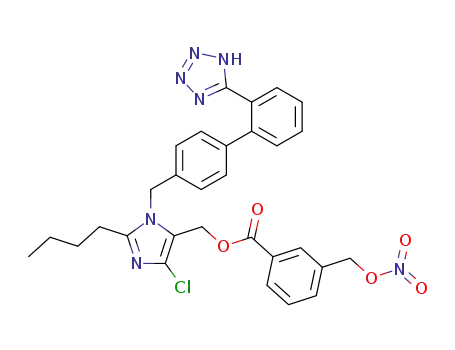
3-nitrooxymethyl-benzoic acid 2-butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-imidazol-4-ylmethyl ester
-
124750-99-8
cozaar
-
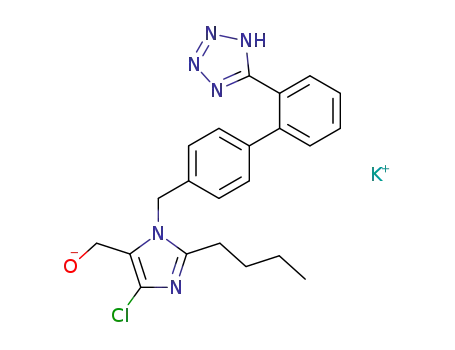
124750-99-8
losartan potassium
-
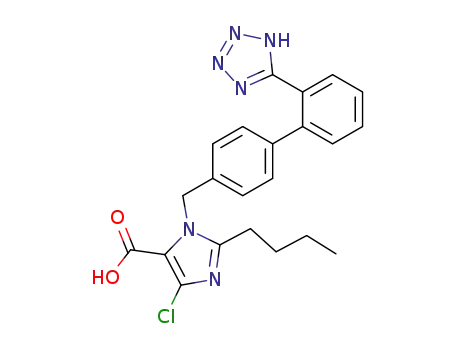
124750-92-1
2-butyl-4-chloro-1-((2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-imidazole-5-carboxylic acid

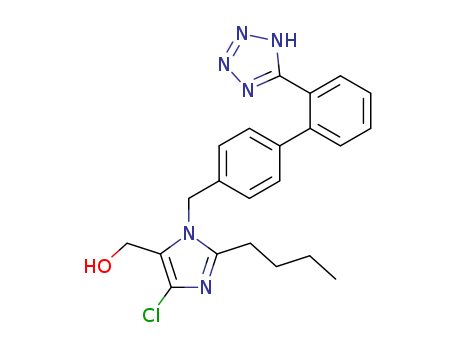
![2-n-butyl-4-chloro-1-[(2'-(2-triphenylmethyl-2H-tetrazol-5-yl)-1,1'-biphenyl-4-yl)methyl]-1H-imidazole-5-methanol](/upload/2025/8/a87f7e70-9a71-440d-a403-e214a9b27118.png)


![2-n-butyl-4-chloro-5-hydroxymethyl-1-[(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]imidazole](/upload/2025/8/16fb9207-1c5f-43de-9255-cbf2fb18f82e.png)
![2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol](/upload/2025/8/0a9d7732-10d2-4f4d-bfee-c87a4ec06f23.png)