|
Chemical Description
|
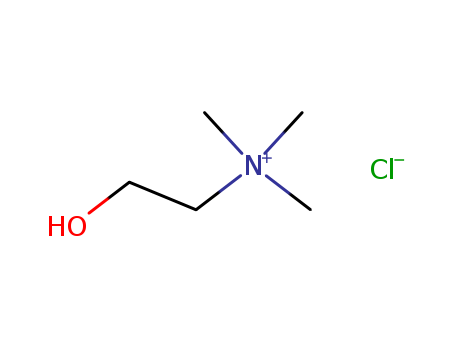
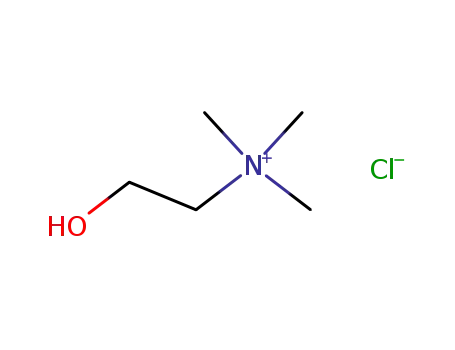
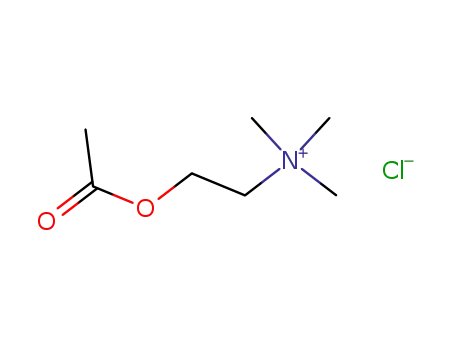
Choline chloride is a quaternary ammonium salt used as a dietary supplement and in the synthesis of other chemicals. |
|
B vitamins
|
Choline is an indispensible fundamental component in humans and animal body, often referred to as B vitamins or vitamin B4, and is a necessary low-molecule organic compound for maintaining physiological function off animal body. It can be synthesized inside animal body but still often need to be supplied to dietary and is a kind of vitamin in maximal usage amount. Inside animal cells, it can be used to adjust the in vivo metabolism and conversion of fats, preventing the fat deposition and tissue degeneration of liver and kidney, and then promote the regeneration of amino acids, enhance utilization of amino acids as well as save some part of methionine.
Choline chloride is the most commonly used as well as most economical form of synthetic choline and is a water soluble vitamin, and is the component for constituting of acetylcholine, lecithin, and nerve phospholipids of biological tissue. Moreover, it can save methionine and is an important material required for livestock, poultry, and fish. Inside animal body, it can be used for adjusting in vivo metabolism and conversion of fats and can prevent the deposition in liver and related tissue degeneration. As a methyl donor, it can promote the re-formation of amino acids and improve the utilization of amino acids. It is mainly used as an additive for being mixing into the animal feed. During the exact usage process, in addition to prevent moisture deliquescence, you should also note that all kind of feeds usually take the addition of choline chloride as the last step. Because of its destruction effects on other vitamins, especially its rapid destruction on vitamin A, D, K in the presence of metal elements, multi-dimensional formulation should not include choline. Daily feed supplied with it should be used as soon as possible after the addition. Tests have showed that choline chloride is especially important for chicken poultry. Its synthetic amino acids and lecithin can be delivered to various locations inside chicken bodies, being able to prevent the fat deposition in the liver and kidney and accelerate the growth of chickens and increase egg production and hatchability.
The above information is edited by the lookchem of Dai Xiongfeng. |
|
Content Analysis
|
Accurately weigh sample of about 300 mg and put it into 250 ml Erlenmeyer flask; add 50 ml of glacial acetic acid and heated on a steam bath until complete dissolving. After cooling, add 10 ml of mercury acetic acid mercury test solution (TS-137) and 2 drops of gentian violet reagent; use the acetic acid solution of 0.1 mol/L perchloric acid for titration to green endpoint. At the same time carry out a blank test and make necessary calibration. Each Ml of 0.1mol/L perchloric acid is equivalent to 13.96 mg of choline chloride (C5 H14ClNO). |
|
Toxicity
|
ADI does not make restrictive regulations (FAO/WHO, 2001).
GRAS (FDA, §182.5252, §182.8252, 2000);
LD50: 9000mg/kg (rat, oral). |
|
Limited use
|
GMP limit (FDA§182.5252.2000); |
|
Production method
|
(1) Continuous method for preparation of choline chloride solution: continuously send the trimethylamine hydrochloride and a certain amount of ethylene oxide separately through pump into the reactor; the reactants had a residence time at the reactor of 1-1.5h; the reaction was carried out under stirring and has its resulting product being continuously withdrawn so that the liquid level within the reactor remained stable. The withdrawn choline chloride extraction crude product entered into the stripper to obtain 60-80% choline chloride liquid product from the bottom.
(2) Trimethylamine hydrochloride was reacted with ethylene oxide, and then added with an organic acid for neutralization and further concentration to obtain the choline chloride (3) Chloro-ethanol was reacted with trimethylamine to generate choline chloride.
(3)Ethylene oxide method. It can be made from the reaction between ethylene oxide and trimethylamine.
Add the trimethylamine ethanol solution into the reactor, send through ethylene oxide at about 30 ℃ and stirring reaction of 4 hour and further obtain it through neutralization with hydrochloric acid (control PH at 6.5-7.0). The yield of the crude product can be as high as 98%. The crude product can further be subject to activated carbon decolorizing and vacuum concentration to obtain 70% aqueous solution. The aqueous solution was added with ground corn cobs, rice hull flour, wheat bran or diatomaceous earth and some other kinds of excipients and can give 50% of the powder.
(4) Chlorohydrin method. Use chlorohydrin to substitute ethylene oxide and hydrochloric acid; have it reacted with trimethylamine in the presence of a small amount of ethylene oxide or alkaline substance;
First add 100 parts of chlorohydrin into the reaction vessel, further add 130 parts of trimethylamine from the liquid surface, while supplying 1.7 parts of ethylene oxide to trigger the reaction. After the addition, stir at 32-38 ℃ for 4h with the yield being 84% (calculated from chlorohydrin). For example, if catalyzed with an alkaline substance (such as quaternary ammonium salts), the one-way conversion rate can reach over 97%.
Trimethylamine methanol solution and chlorohydrin is subject to heating reaction, concentration under reduced pressure, and re-crystallization to generate it. |
|
Reactivity Profile
|
An acidic organic salt. Materials in this group are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. |
|
Fire Hazard
|
Choline chloride is relatively nonflammable. |
|
Biochem/physiol Actions
|
The enzymatic activities of butyrylcholinesterase (BChE) and paraoxonase 1 (PON1), two serum enzymes synthesized by the liver and related with inflammation, were decreased in a sepsis animal model injected with LPS. Choline chloride administered intravenously at 20 mg/kg body weight prevents the LPS-mediated decreases in the activities of these two enzymes . |
|
Safety Profile
|
Poison by
intraperitoneal and intravenous routes.
Moderately toxic experimentally by ingestion
and subcutaneous routes. Mutation data
reported. A lipotropic agent which induces
the reduction in fats contained in the liver.
When heated to decomposition it emits
toxic fumes of Cl-, SOx and NOx. See also
CHOLINE. |
|
Purification Methods
|
Extremely deliquescent. Check purity by AgNO3 titration or by titration of OH-base after passage through an anion-exchange column. Crystallise it from absolute EtOH, or EtOH/Et2O, dry it under vacuum and store it in a vacuum desiccator over P2O5 or Mg(ClO4)2. [Beilstein 4 IV 1443.] |
|
Chemical Composition
|
Choline chloride is a quaternary ammonium salt composed of choline cation and chloride anion. It contains a choline moiety. |
|
Role as an Animal Growth Promotant
|
Choline chloride serves as an animal growth promotant, implying its significance in enhancing animal growth and performance. |
|
Use in Deep Eutectic Solvents (DESs)
|
Choline chloride is a key component in the formation of deep eutectic solvents (DESs), which are solvents with properties similar to ionic liquids (ILs) but are easily prepared by mixing low-cost components.
DESs are formed through hydrogen bonding interactions between components, resulting in a mixture with a much lower melting point than individual components.
Choline chloride's affordability, biodegradability, and non-toxic nature make it a popular choice for DES synthesis. |
|
Applications of DESs
|
DESs serve as a greener alternative to ILs and find applications in various fields, including synthesis, metal-catalyzed organic reactions, electrochemistry, nanomaterials, biochemistry, separation, and analysis. |
|
Effect on Delignification
|
Chloride anion, an active component of choline chloride, contributes to the delignification process.
Choline chloride enhances the cleavage rate of 尾-O-4 bonds in lignin, thereby increasing the delignification rate of biomass, particularly in the case of Eucalyptus.
Studies suggest that choline chloride is effective in increasing the delignification rate when used in conjunction with lactic acid, with chloride anion being the active component.
Despite its effectiveness, choline chloride is not significantly superior to inexpensive salts like NaCl in this regard. |
|
Effect on Lignin Solubility and Mass Transfer
|
Choline chloride has been found to slightly decrease the solubility of lignin in DESs. Additionally, it increases viscosity, leading to a decrease in the estimated mass transfer coefficient. |
|
General Description
|
Choline chloride is a quaternary ammonium salt and an essential nutrient that functions as a precursor for acetylcholine and phospholipids, playing a critical role in liver function, brain development, and lipid metabolism. It is widely used in animal feed as a dietary supplement to promote growth and improve feed efficiency, as well as in human nutrition for its potential cognitive and hepatic benefits. Additionally, it serves as an industrial chemical in applications such as a clay stabilizer in oil drilling and a choline source in pharmaceutical formulations. Its hygroscopic nature and solubility in water make it suitable for various liquid and solid formulations. |
|
Application
|
Choline chloride has been used:as a component of choline acetyltransferase (ChAT) buffer and for choline uptake in cultured human neuroblastoma (SK-N-SH) cellsfor choline standard curve generation for quantifying phospholipase D (PLD) activityas a component of slice solution for the dissection of entorhinal-hippocampal brain slices |
|
Definition
|
ChEBI: Choline chloride is a quaternary ammonium salt with choline cation and chloride anion. It has a role as an animal growth promotant. It is a chloride salt and a quaternary ammonium salt. It contains a choline. |