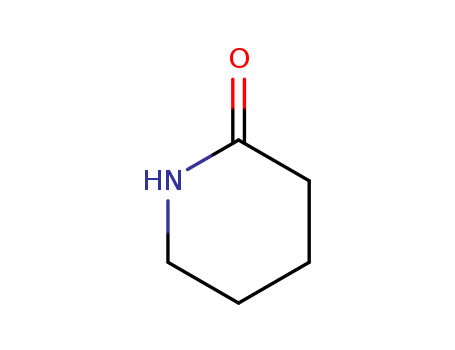
Cost-effective and customizable 2-Piperidone Manufacturer 675-20-7 supplier
- Molecular Formula:C5H9NO
- Molecular Weight:99.1326
- Appearance/Colour:white to yellowish low melting crystalline mass
- Vapor Pressure:0.0158mmHg at 25°C
- Melting Point:38-40 °C(lit.)
- Refractive Index:1.448
- Boiling Point:256 °C at 760 mmHg
- Flash Point:133.1 °C
- PSA:29.10000
- Density:1.001 g/cm3
- LogP:0.61530
2-Piperidone(Cas 675-20-7) Usage
|
Preparation
|
2-Piperidone can be obtained from cyclopentanone as raw material by a two-step reaction. |
|
Synthesis Reference(s)
|
Journal of the American Chemical Society, 102, p. 7629, 1980 DOI: 10.1021/ja00546a001Tetrahedron Letters, 28, p. 2829, 1987 DOI: 10.1016/S0040-4039(00)96220-8The Journal of Organic Chemistry, 21, p. 965, 1956 DOI: 10.1021/jo01115a010 |
|
Purification Methods
|
Purify it by repeated fractional distillation.[Cowley J Org Chem 23 1330 1958, Reppe et al. Justus Liebigs Ann Chem 596 198 1955, IR: Huisgen et al. Chem Ber 90 1437 1957.] The hydrochloride has m 183-184o (from isoPrOH or EtOH/Et2O) [Hurd et al. J Org Chem 17 865 1952], and the oxime has m 122.5o (from pet ether) [Behringer & Meier Justus Liebigs Ann Chem 607 67 1957]. The picrate has m 92-93o. [Beilstein 21 H 239, 21 III/IV 3170, 21/6 V 396.] |
|
Definition
|
ChEBI: 2-Piperidone is a delta-lactam that is piperidine which is substituted by an oxo group at position 2. It has a role as an EC 1.2.1.88 (L-glutamate gamma-semialdehyde dehydrogenase) inhibitor. It is a member of piperidones and a delta-lactam. |
InChI:InChI=1/C5H9NO/c7-5-3-1-2-4-6-5/h1-4H2,(H,6,7)
675-20-7 Relevant articles
The benzyl can be selectively removed by visible light or near visible light. Method for protecting allyl and propargyl group
-
Paragraph 0010, (2021/10/16)
The invention provides a method for sele...
PRODUCTION METHOD OF CYCLIC COMPOUND
-
Paragraph 0057; 0059; 0062; 0064, (2021/05/05)
PROBLEM TO BE SOLVED: To provide an indu...
An Integrated Cofactor/Co-Product Recycling Cascade for the Biosynthesis of Nylon Monomers from Cycloalkylamines
Sarak, Sharad,Sung, Sihyong,Jeon, Hyunwoo,Patil, Mahesh D.,Khobragade, Taresh P.,Pagar, Amol D.,Dawson, Philip E.,Yun, Hyungdon
, p. 3481 - 3486 (2020/12/17)
We report a highly atom-efficient integr...
En Route to a Heterogeneous Catalytic Direct Peptide Bond Formation by Zr-Based Metal-Organic Framework Catalysts
Conic, Dragan,De Azambuja, Francisco,Harvey, Jeremy N.,Loosen, Alexandra,Parac-Vogt, Tatjana N.,Van Den Besselaar, Maxime
, p. 7647 - 7658 (2021/06/30)
Peptide bond formation is a challenging,...
675-20-7 Process route
-
-
505-18-0
2,3,4,5-tetrahydropyridine
Conditions
| Conditions |
Yield |
|
piperidine;
With
chloroamine; sodium hydroxide;
In
water;
at 25 ℃;
pH=12.89;
With
water;
|
|
-
-
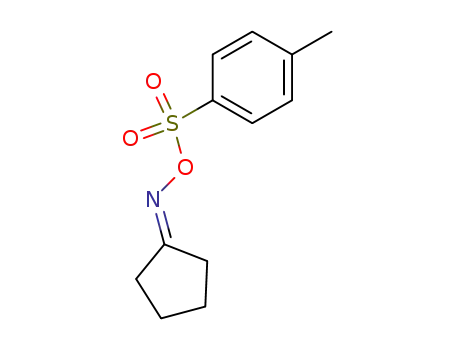
10442-97-4
cyclopentanone oxime tosylate
-
-
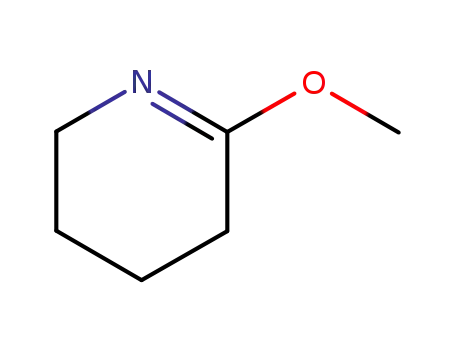
5693-62-9
2,3,4,5-tetrahydro-6-methoxypyridine
675-20-7 Upstream products
675-20-7 Downstream products
-
1147-76-8
5-(N-phthalimido)pentanoic acid
-
3326-13-4
N-acetyl-2-piperidone
-
2446-62-0
7,8,9-trihydropyrido[2,1-b]quinazolin-11(6H)-one
-
13070-01-4
piperidine-2-thione