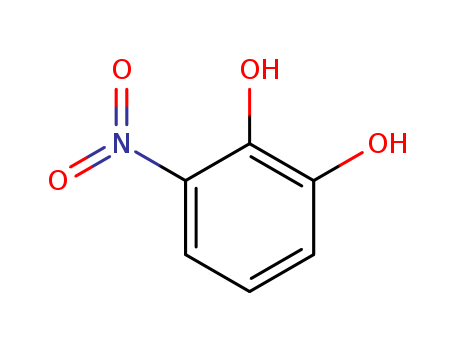
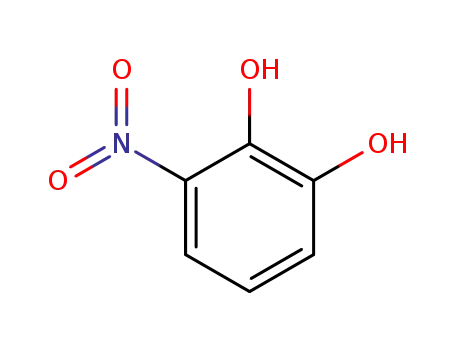
Buy cost-effective 99% pure 3-Nitrocatechol 6665-98-1 now
- Molecular Formula:C6H5 N O4
- Molecular Weight:155.11
- Vapor Pressure:0.00461mmHg at 25°C
- Refractive Index:1.5423 (estimate)
- Boiling Point:268.6°C at 760 mmHg
- Flash Point:123.7°C
- PSA:86.28000
- Density:1.58g/cm3
- LogP:1.52920
3-Nitrocatechol(Cas 6665-98-1) Usage
InChI:InChI=1/C6H5NO4/c8-5-3-1-2-4(6(5)9)7(10)11/h1-3,8-9H
6665-98-1 Relevant articles
Gas-phase IR cross-sections and single crystal structures data for atmospheric relevant nitrocatechols
Arsene, Cecilia,Bejan, Iustinian-Gabriel,Olariu, Romeo-Iulian,Roman, Claudiu,Roman, Tiberiu
, (2021/09/28)
The gas-phase IR absorption cross sectio...
Cystobactamid 507: Concise Synthesis, Mode of Action, and Optimization toward More Potent Antibiotics
Elgaher, Walid A. M.,Hamed, Mostafa M.,Baumann, Sascha,Herrmann, Jennifer,Siebenbürger, Lorenz,Krull, Jana,Cirnski, Katarina,Kirschning, Andreas,Br?nstrup, Mark,Müller, Rolf,Hartmann, Rolf W.
supporting information, p. 7219 - 7225 (2020/05/08)
Lack of new antibiotics and increasing a...
Class of large ring heterocyclic compound restraining HCV and manufacturing and uses thereof
-
Paragraph 0278; 0279; 0280; 0281, (2017/09/01)
The invention relates to a class of comp...
Microwave assisted synthesis of nitro phenols from the reaction of phenols with urea nitrate under acid-free conditions
Verma, Sanny,Pandita, Sangeeta,Jain, Suman L.
, p. 1320 - 1322 (2014/03/21)
Urea nitrate was found to be an inexpens...
6665-98-1 Process route
-
-
622-42-4
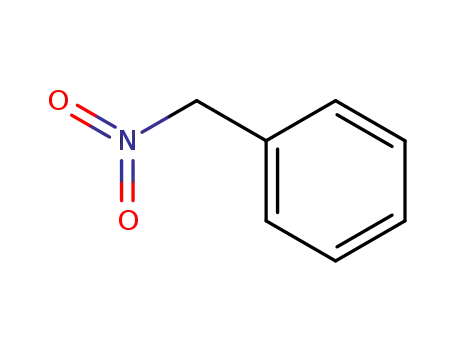
1-nitro-1-phenylmethane
-
-
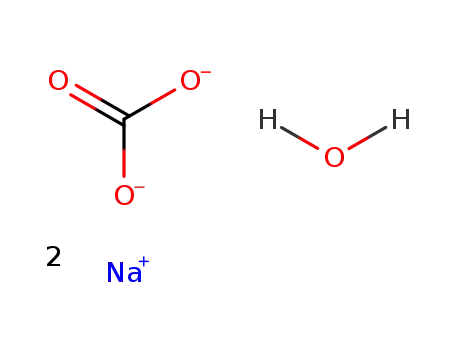
sodium carbonate monohydrate
-
-
6665-98-1
3-nitrobenzene-1,2-diol
-
-
5428-02-4
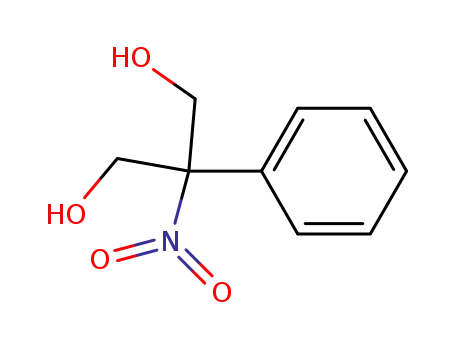
2-nitro-2-phenyl-propane-1,3-diol
Conditions
| Conditions |
Yield |
|
With
formaldehyd;
In
(2S)-N-methyl-1-phenylpropan-2-amine hydrate; toluene;
|
78%
|
|
With
formaldehyd;
In
(2S)-N-methyl-1-phenylpropan-2-amine hydrate; toluene;
|
78%
|
|
With
formaldehyd;
In
(2S)-N-methyl-1-phenylpropan-2-amine hydrate; toluene;
|
78%
|
-
-
120-80-9,19481-10-8,37349-32-9
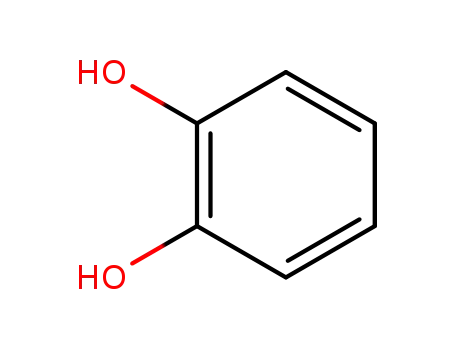
benzene-1,2-diol
-
-
6665-98-1
3-nitrobenzene-1,2-diol
Conditions
| Conditions |
Yield |
|
With
uronium nitrate;
In
water; acetonitrile;
at 80 ℃;
for 0.5h;
regioselective reaction;
Microwave irradiation;
|
75%
|
|
With
copper(II) nitrate/zeolite H-Y;
at 70 - 80 ℃;
for 2h;
Further Variations:;
heating mode; times;
Product distribution;
|
65%
|
|
With
nitric acid;
In
diethyl ether;
at 0 - 20 ℃;
for 24h;
Cooling with ice;
|
50%
|
|
With
nitric acid;
In
diethyl ether;
at 0 - 20 ℃;
|
31.2%
|
|
With
nitric acid;
In
diethyl ether;
at 0 - 20 ℃;
for 0.333333h;
|
30%
|
|
With
diethyl ether; nitric acid;
|
|
|
durch Nitrierung;
|
|
|
Multi-step reaction with 3 steps
1: aqueous sodium carbonate-solution; sodium dithionite
2: aqueous nitric acid; acetic acid / 30 - 35 °C
3: ethanolic KOH
With
potassium hydroxide; sodium dithionite; nitric acid; sodium carbonate; acetic acid;
|
|
|
With
conc. nitric acid;
In
Petroleum ether;
|
|
|
With
nitric acid;
|
|
|
With
nitric acid;
In
diethyl ether;
|
|
|
With
nitric acid;
In
diethyl ether;
at 20 ℃;
for 0.333333h;
|
|
|
With
nitric acid;
In
diethyl ether;
for 0.5h;
|
|
6665-98-1 Upstream products
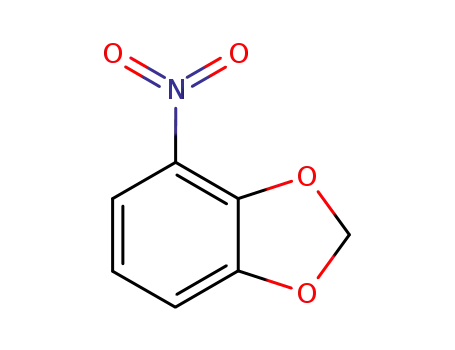
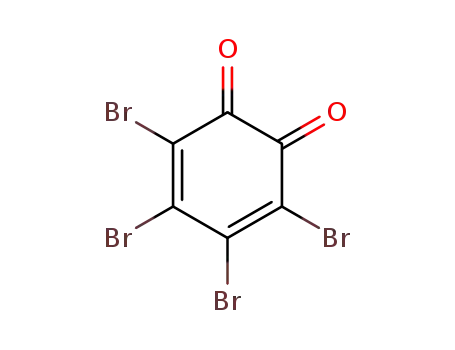
6665-98-1 Downstream products
-
72744-45-7
4-nitro-(1,3-benzodioxolyl)
-
2435-54-3
o-bromanil
-
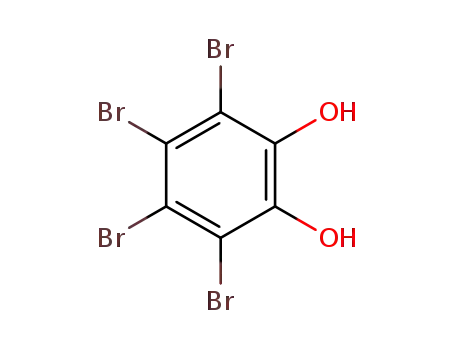
488-47-1
Tetrabromocatechol
-
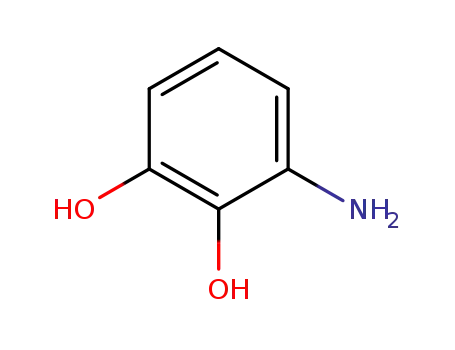
20734-66-1
1,2-dihydroxy-3-aminobenzene