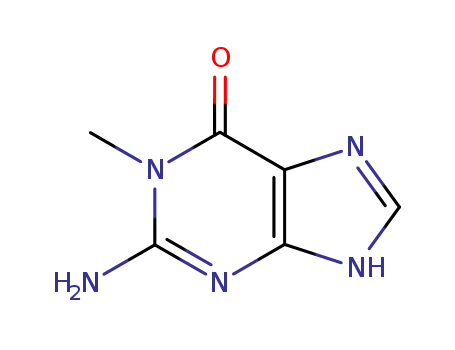
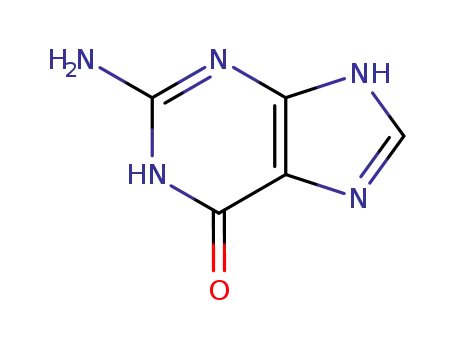
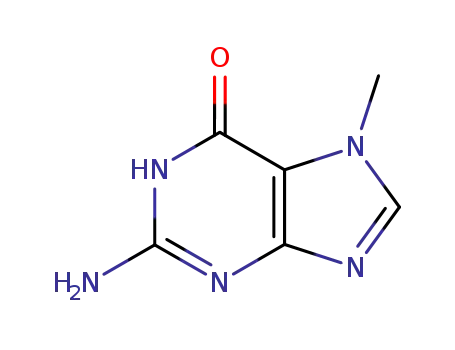
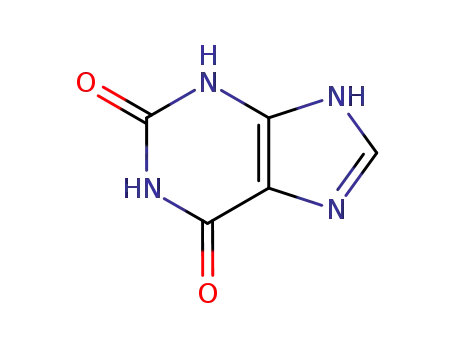
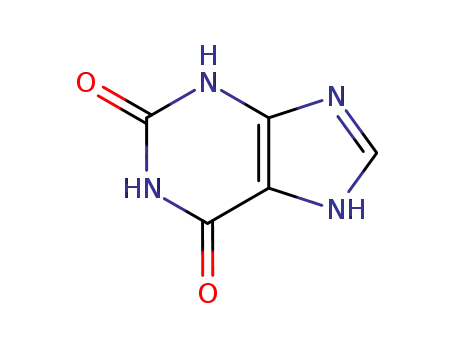
Good factory exports good 2,6-Dihydroxypurine 69-89-6
- Molecular Formula:C5H4N4O2
- Molecular Weight:152.112
- Appearance/Colour:White to off-white crystalline powder
- Melting Point:300 °C
- Refractive Index:1.989
- Boiling Point:834.9 °C at 760 mmHg
- PKA:pKa 9.95 (Uncertain)
- Flash Point:458.7 °C
- PSA:94.40000
- Density:1.94 g/cm3
- LogP:-1.06050
2,6-Dihydroxypurine(Cas 69-89-6) Usage
|
Synthesis Reference(s)
|
Chemical and Pharmaceutical Bulletin, 22, p. 1200, 1974 DOI: 10.1248/cpb.22.1200 |
|
Biochem/physiol Actions
|
A high level of xanthine is implicated in metabolic disorders like Lesch-Nyhan syndrome. A xanthine-based biosensor may be useful for detecting xanthine in food and clinical samples. Blood and urine samples of patients with renal failure, gout and xanthinuria show high levels of xanthine. |
|
Purification Methods
|
The monohydrate separates in a microcrystalline form on slow acidification with acetic acid of a solution of xanthine in dilute NaOH. It is also precipitated by addition of conc NH3 to its solution in hot 2N HCl (charcoal). After washing with H2O and EtOH, it is dehydrated by heating above 125o. Its solubility in H2O is 1 in 14,000parts at 16o and 1 in 1,500parts of boiling H2O, and separates as plates . It has no m, but the perchlorate has m 262-264o [Lister Purines Part II, Fused Pyrimidines Brown Ed, Wiley-Interscience pp252-253 1971, ISBN 0-471-38205-1]. [Beilstein 26 H 447, 26 I 131, 26 II 260, 26 III/IV 2327.] |
|
Definition
|
A poisonous colorless
crystalline organic compound that occurs
in blood, coffee beans, potatoes, and urine.
It is used as a chemical intermediate. |
|
General Description
|
Xanthine is a purine that can be produced in the purine metabolic pathway via different precursors:Guanine deamination by guanine deaminaseHypoxanthine conversion by xanthine oxidoreductase |
InChI:InChI=1/C5H2N4O2/c10-4-2-3(7-1-6-2)8-5(11)9-4/h1H,(H,9,10,11)
69-89-6 Relevant articles
Label-Free Surface Enhanced Raman Scattering Approach for High-Throughput Screening of Biocatalysts
Westley, Chloe,Xu, Yun,Carnell, Andrew J.,Turner, Nicholas J.,Goodacre, Royston
, p. 5898 - 5903 (2016)
Biocatalyst discovery and directed evolu...
Identification of function and mechanistic insights of guanine deaminase from Nitrosomonas europaea: Role of the C-terminal loop in catalysis
Bitra, Aruna,Hussain, Bhukya,Tanwar, Ajay Singh,Anand, Ruchi
, p. 3512 - 3522 (2013)
NE0047 from Nitrosomonas europaea has be...
-
Herak,Gordy
, p. 1287 (1965)
-
-
Birdall et al.
, p. 3,-13 (1972)
-
The Influencing of Preanodized Inlaying Ultrathin Carbon Paste Electrode on the Oxidation for the Xanthine and Hypoxanthine by the Hydrogen Bond
Qiao, Yue-Chun,Li, Jing,Li, Yao,Li, Quan-Min
, p. 1011 - 1019 (2015)
In this paper, a pre-anodized inlaying u...
Real-Time Monitoring of Human Guanine Deaminase Activity by an Emissive Guanine Analog
Bucardo, Marcela S.,Wu, You,Ludford, Paul T.,Li, Yao,Fin, Andrea,Tor, Yitzhak
, p. 1208 - 1214 (2021/07/26)
Guanine deaminase (GDA) deaminates guani...
Inhibition of xanthine oxidase by theaflavin: Possible mechanism for anti-hyperuricaemia effect in mice
Chen, Jianmin,Jin, Nan,Li, Qinglian,Ran, Mengnan,Ruan, Zhipeng,Ye, Yaling
, p. 11 - 18 (2020/07/03)
Xanthine oxidase (XO) catalyzes the oxid...
SUBSTITUTED XANTHINE DERIVATIVES
-
Page/Page column 41, (2020/07/07)
The present invention relates to compoun...
One-Step Synthesis of 2-Fluoroadenine Using Hydrogen Fluoride Pyridine in a Continuous Flow Operation
Salehi Marzijarani, Nastaran,Snead, David R.,McMullen, Jonathan P.,Lévesque, Fran?ois,Weisel, Mark,Varsolona, Richard J.,Lam, Yu-Hong,Liu, Zhijian,Naber, John R.
supporting information, p. 1522 - 1528 (2019/07/10)
We report the development of a one-pot s...
69-89-6 Process route
-
-
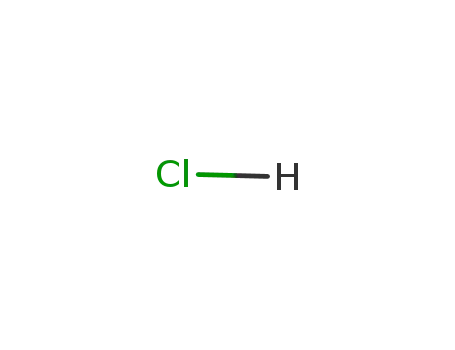
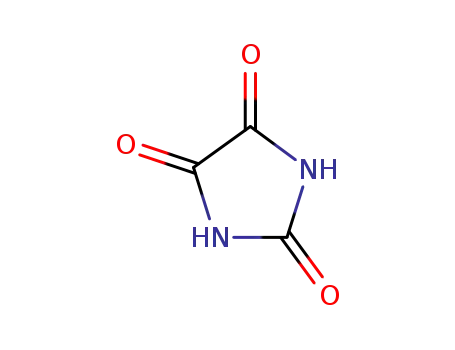
7647-01-0,15364-23-5
hydrogenchloride
-
-
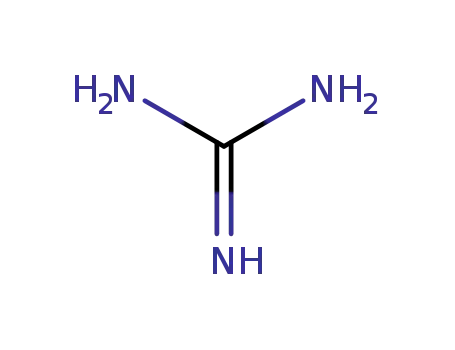
113-00-8
guanidine nitrate
-
-
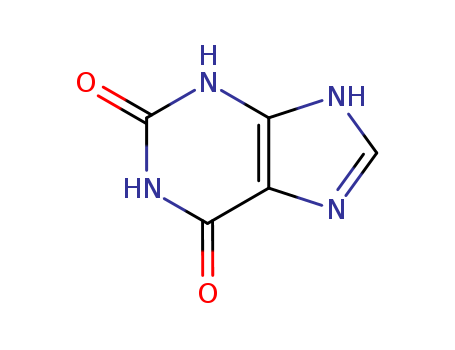
69-89-6,6053-41-4
xanthin
-
-
1774-47-6
trimethylsulfoxonium iodide
-
-
81980-39-4
Carbamic acid (1S,2R)-2,7-diamino-1-[9-(4-hydroxy-5-phosphonooxymethyl-tetrahydro-furan-2-yl)-6-oxo-6,9-dihydro-1H-purin-2-ylamino]-6-methyl-5,8-dioxo-2,3,5,8-tetrahydro-1H-pyrrolo[1,2-a]indol-9-ylmethyl ester
-
-
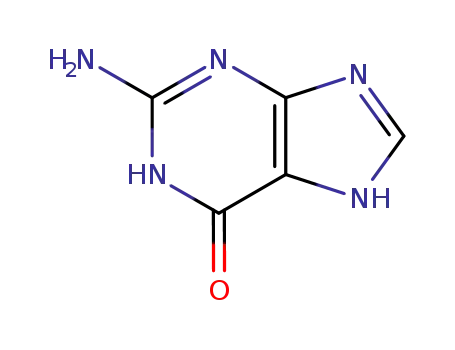
73-40-5
2-amino-1,9-dihydro-6H-purin-6-one
-
-
2958-98-7
3-methylguanine
Conditions
| Conditions |
Yield |
|
With
hydrogenchloride; hydroxy-1-propyne;
In
dimethyl sulfoxide;
at 100 ℃;
for 1h;
Product distribution;
|
10%
1%
3%
5%
4%
|
69-89-6 Upstream products
69-89-6 Downstream products
-
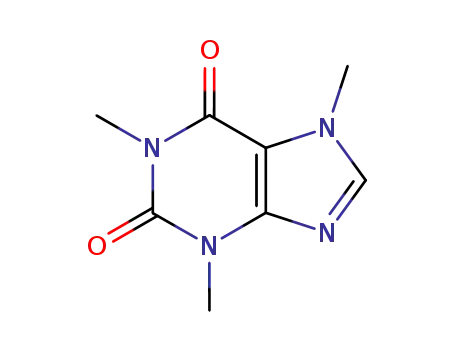
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione
-
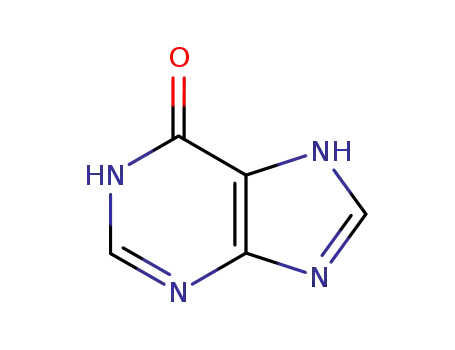
68-94-0
1,7-dihydro-6H-purin-6-one
-
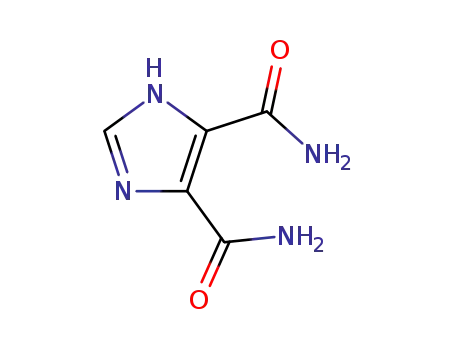
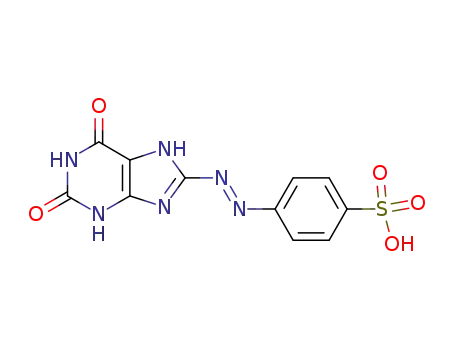
75056-38-1
4-(2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-ylazo)-benzenesulfonic acid
-
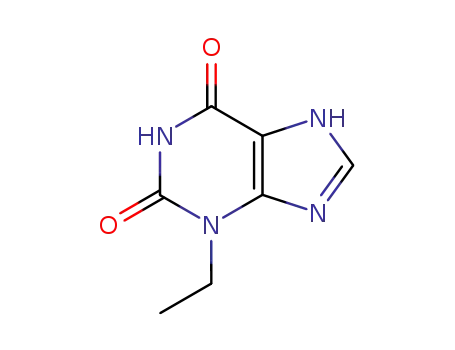
41078-01-7
3-Ethylxanthine



![Carbamic acid (1S,2R)-2,7-diamino-1-[9-(4-hydroxy-5-phosphonooxymethyl-tetrahydro-furan-2-yl)-6-oxo-6,9-dihydro-1H-purin-2-ylamino]-6-methyl-5,8-dioxo-2,3,5,8-tetrahydro-1H-pyrrolo[1,2-a]indol-9-ylmethyl ester](/upload/2025/8/3a1cd62d-f385-4f12-a98e-f8ca101273c9.png)