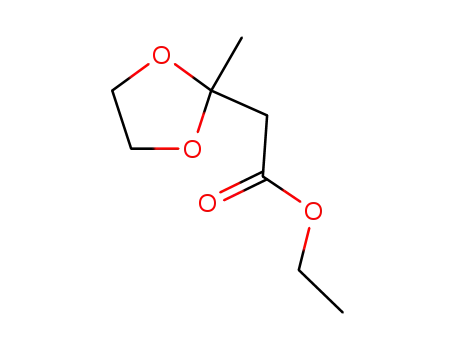
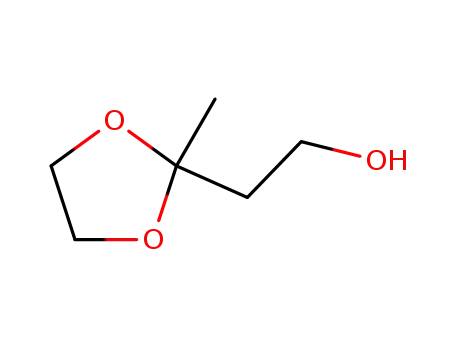
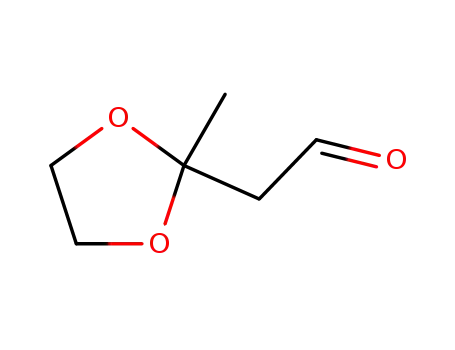
| Conditions |
Yield |
|
With
montmorillonite K-10;
In
benzene;
for 6h;
Heating;
|
98%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 48h;
Reflux;
|
98.6%
|
|
With
Amberlyst15;
In
cyclohexane;
Reagent/catalyst;
Solvent;
Reflux;
|
97%
|
|
With
boron trifluoride diethyl etherate;
In
dichloromethane;
at 25 ℃;
for 48h;
|
96%
|
|
USY-3 zeolite;
In
toluene;
at 145.85 ℃;
for 1h;
|
96%
|
|
With
toluene-4-sulfonic acid;
|
96%
|
|
With
chitosan immobilized with 3-(3-vinylimidazolyl)propane-1-sulfonate and 12-phosphotungstic acid;
In
cyclohexane;
at 80 ℃;
for 2h;
Time;
Reflux;
Dean-Stark;
|
96.2%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 17h;
Heating;
|
95%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 24h;
Heating;
|
93%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
Heating;
|
92%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 8h;
Heating;
|
91%
|
|
With
toluene-4-sulfonic acid;
In
toluene;
Reflux;
|
91%
|
|
With
polyaniline-sulfate salt;
In
toluene;
for 1.5h;
Heating;
|
90%
|
|
With
toluene-4-sulfonic acid;
|
90%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 8h;
Heating;
|
90%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 5h;
Heating;
|
90%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 20h;
Reflux;
|
88%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 24h;
Dean-Stark;
Reflux;
|
87%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 8h;
Heating;
|
86%
|
|
With
toluene-4-sulfonic acid;
In
toluene;
for 4h;
Reflux;
|
83%
|
|
With
toluene-4-sulfonic acid;
In
toluene;
for 12h;
Heating;
|
80%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
Heating;
|
75%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 20h;
Heating;
|
73%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 24h;
Heating;
|
70%
|
|
With
N,N,N-triethyl-N-butanesulfonic acid ammonium hydrogen sulfate;
at 79.99 ℃;
for 2h;
Temperature;
Concentration;
Reagent/catalyst;
Time;
|
61.6%
|
|
With
toluene-4-sulfonic acid;
In
benzene;
Heating;
|
58%
|
|
With
toluene-4-sulfonic acid;
In
toluene;
for 5h;
Dean-Stark;
Heating;
|
54%
|
|
|
|
|
With
toluene-4-sulfonic acid;
|
|
|
With
toluene-4-sulfonic acid;
In
benzene;
|
|
|
|
|
|
With
toluene-4-sulfonic acid;
In
benzene;
Heating;
|
|
|
With
carbon-based acid prepared by carbonization of furaldehyde and 2-hydroxyethylsulfonic acid;
In
cyclohexane;
for 2.5h;
Reflux;
|
|
|
With
tin(II) hydroxide chloride;
In
neat (no solvent);
at 100 ℃;
for 2h;
under 760.051 Torr;
Reagent/catalyst;
|
|
|
With
toluene-4-sulfonic acid;
In
toluene;
|
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 20h;
Reflux;
|
|
|
With
toluene-4-sulfonic acid;
In
toluene;
for 2h;
Dean-Stark;
Reflux;
|
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 48h;
Reflux;
|
|
|
With
acidic PO3H-functionalized cellulose;
In
neat (no solvent);
at 120 ℃;
for 6h;
Reagent/catalyst;
Solvent;
chemoselective reaction;
Green chemistry;
|
|

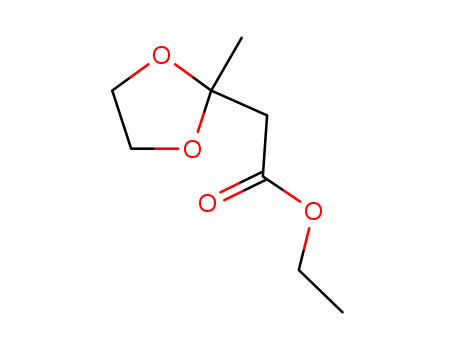
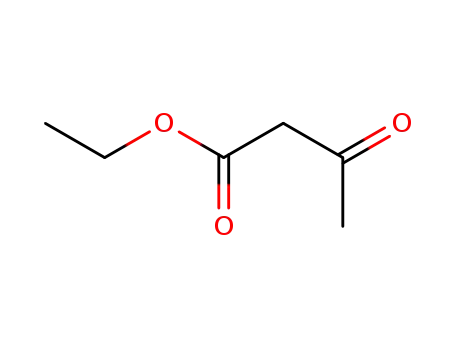

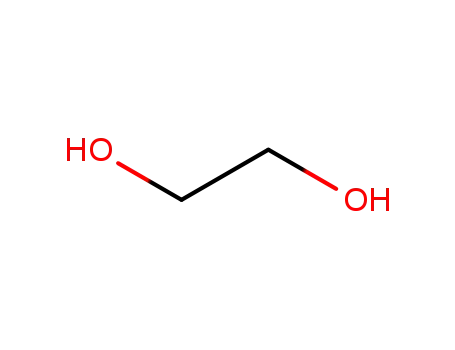

![(2-methyl-[1,3]dioxolan-2-yl)-acetic acid](/upload/2025/8/ae5f9906-26b5-4fe0-866c-fd75efb38c6a.png)